BAR proteins - membrane curvature sensing and deformation
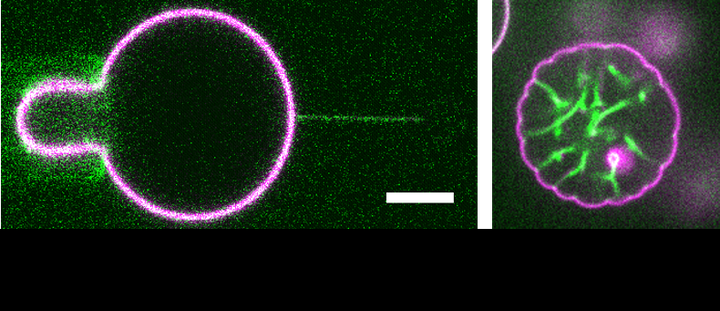 Left: BAR proteins are sorted in the highly curved membrane tubes compared to the relatively flat GUV membranes. Right: At low bulk concentration, the I-BAR domain of IRSp53 self-organizes linearly and deforms GUV membranes, resulting in GUVs with flower-like shape. Green: BAR protein, Magenta: Membrane
Left: BAR proteins are sorted in the highly curved membrane tubes compared to the relatively flat GUV membranes. Right: At low bulk concentration, the I-BAR domain of IRSp53 self-organizes linearly and deforms GUV membranes, resulting in GUVs with flower-like shape. Green: BAR protein, Magenta: MembraneBAR domain containing proteins have been well-recognized to be involved in numerous cellular processes, such as endocytosis and intracellular trafficking, where the cellular membranes undergo remarkable shape change. Due to their crescent shape, BAR domains have a high propensity to bind to curved membranes matching their intrinsic curvature, which contributes to their recruitment at highly curved membrane regions in cells. Also, BAR domains can deform membranes, which contributes to membrane reshaping in cells. Yet, a comprehensive mechanistic description of how BAR proteins sense and generate membrane curvature is missing. We aim to reveal the mechanisms underlying the membrane curvature sensing and deformation abilities of BAR proteins by using biophysical approaches, such as membrane nanotube pulling using optical tweezers, and bottom-up reconstitution systems composed of GUVs and purified BAR proteins.
Related publications