Filopodia formation
 Left Filopodia generated by overexpressing the I-BAR domain of IRSp53 (shown in magenta). Actin-membrane linker Ezrin (shown in cyan) is enriched in these filopodia.
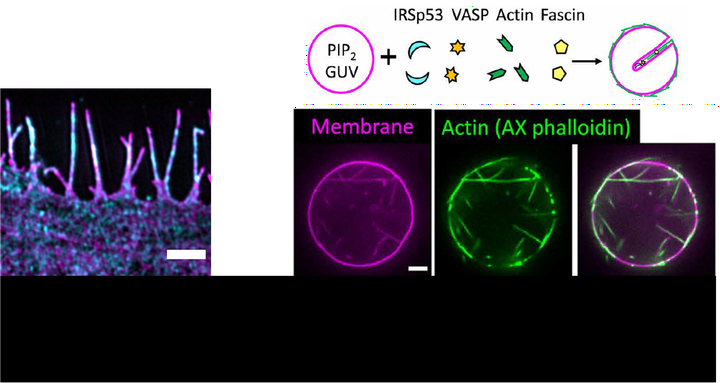
Right IRSp53 and VASP synergistically drive the formation of inward actin-filled membrane protrusions on GUVs.
Left Filopodia generated by overexpressing the I-BAR domain of IRSp53 (shown in magenta). Actin-membrane linker Ezrin (shown in cyan) is enriched in these filopodia.
Right IRSp53 and VASP synergistically drive the formation of inward actin-filled membrane protrusions on GUVs.Scientific question Filopodia are actin-filled finger-like membrane protrusions that are important for cell function, including migration and exploration of the environment, but their formation and regulation mechanisms are not fully understood. Cell biology studies have shown that the curvature-sensing protein IRSp53 promotes filopodia formation by interacting with VASP, which enhances actin polymerization. However, the underlying molecular mechanism of IRSp53-driven filopodia formation remains elusive.
Our Contribution We have developed in vitro reconstitution assays to demonstrate the ability of the curvature-sensing protein IRSp53 to orchestrate local actin assembly on membranes, leading to the formation of membrane tubes. The in vitro assays use giant unilamellar vesicles (GUVs) as model membranes and purified IRSp53, VASP, actin and fascin. We demonstrated that IRSp53 clusters recruit VASP to assemble actin filaments on membranes, generating actin-filled protrusions that resemble filopodia. We found that IRSp53 is enriched and induces actin assembly only in highly dynamic membrane regions of nanotubes pulled from living cells.
Impact Our findings advance the understanding of a fundamental question in biophysics and cell biology: how cells initiate actin-driven protrusions, such as filopodia, at specific membrane locations. Our work supports a regulatory mechanism of IRSp53 for precise spatio-temporal control of filopodia initiation and stabilization.
Related publications